Allergy shots (SCIT)
Subcutaneous immunotherapy, also known as allergy shots or SCIT, is a form of long-term treatment that decreases symptoms for many people with allergic rhinitis, allergic asthma, conjunctivitis (eye allergy) or stinging insect allergy. Allergy shots decrease sensitivity to allergens and often lead to lasting relief of allergy symptoms even after treatment is stopped.1,2
Anaphylaxis risk
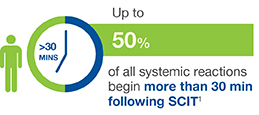
Although the risk is low when SCIT is administered appropriately, life-threatening reactions do occur. Prevalence of severe reactions ranges from 1% for conventional immunotherapy to more than 34% for rush immunotherapy.1
Monitoring guidelines for anaphylaxis
Symptoms of an anaphylactic reaction can include swelling in the throat, wheezing or tightness in the chest, nausea and dizziness. Most serious reactions develop within 30 minutes of the allergy injections. This is why it is recommended that patients wait in their doctor's office for at least 30 minutes after they receive allergy shots, even though evidence shows that not all practices follow this guideline.1,2,5
Self-administered immunotherapy
Another form of allergy immunotherapy, called sublingual immunotherapy (SLIT), was approved in the United States. Rather than shots, SLIT involves administering the allergens in a liquid or tablet form under the tongue, usually on a daily basis.1,6
The US Food and Drug Administration (FDA) has approved four allergy tablet products. Allergy tablets do not need to be given in a medical setting after the first dose. However, the FDA-approved product information for the four SLIT tablets includes a warning about the possibility of severe allergic reactions from SLIT and a recommendation that an epinephrine auto-injector be prescribed to patients receiving allergy tablets, in the event a severe allergic reaction should occur.6,7
Special Offers
Viatris offers Access and Savings Programs for eligible patients. See Terms and Conditions.
There’s only one recommended first-line treatment for anaphylaxis.
heading
Important Safety Information (the following information applies to both EPIPEN and its Authorized Generic)
EPIPEN (epinephrine injection, USP) 0.3 mg and EPIPEN JR (epinephrine injection, USP) 0.15 mg Auto-Injectors are intended for immediate administration as emergency supportive therapy only and are not intended as a substitute for immediate medical or hospital care. In conjunction with the administration of epinephrine, the patient should seek immediate medical or hospital care. More than two sequential doses of epinephrine should only be administered under direct medical supervision.
Rare cases of serious skin and soft tissue infections have been reported following epinephrine injection.
References
- Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(1 Suppl):S1-S55.
- American Academy of Allergy, Asthma & Immunology. Allergy Shots (Immunotherapy). Updated November 13, 2023. Accessed March 14, 2024. https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/allergy-shots-%28immunotherapy%29
- EPIPEN (epinephrine) injection. Prescribing & Patient Information. 2023. Mylan Specialty L.P. Morgantown WV.
- EPINEPHRINE (epinephrine) injection. Prescribing & Patient Information. 2023. Mylan Specialty L.P. Morgantown WV.
- Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008-2012. J Allergy Clin Immunol Pract. 2014;2(2):161-167.
- American Academy of Allergy, Asthma & Immunology. SLIT Treatment for Allergic Rhinitis Nothing to Sneeze About. Reviewed September 28, 2020. Accessed March 14, 2024. https://www.aaaai.org/tools-for-the-public/conditions-library/allergies/slit-treatment-for-allergic-rhinitis-nothing-to-sn
- Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organizations Journal. 2014;7:6.

This site is intended for US healthcare professionals.
If you are a patient, please check out our site for patients.

Prescribing Information
Please select from the following:

Patient Information
Please select from the following: